In this section we will see how chemistry create our daily hair product, from shampoo to hair spray. Besides that, we will see the chemical compounds that make the function of the hair care product, and to begin, we start from a brief overview about hair care products.
Hair Colouring Product
In 1907, French chemist Eugène Schueller created the first 'safe' ceommercial hair colouring formulation based on p-phenylenediamine or PPD (later on, we will see how 'safe' it is). His invention led to the foundation of the French Harmless Hair Dye Company which later became L'Oreal.
According to the Market Leader, L'Oreal shares 16% of a $100 billion per annum global market an invests 5-6% of its turnover in R&D (research and development) approximately $1 billion per annum. Moreover, 1900 of 52000 global workforce are scientists and it has 17 global brands (L'Oreal, Garnier, Maybelline, etc.).
Hair Structure
Before we see the chemical inside hair dye and how it works, it is better to have a quick view about hair structure first.
 |
| The hair structure |
Generally, around 105 hairs per head with the rate of grow in average is 0.4 mm/day, and the life cycle of hair is 5-7 year. Besides that, 70 μm in diameter with the range from 50-100 μm, so the total surface area that is covered by hair on the head is around 6.6 m2.
 |
| The disulphide bridge |
In structure of hair, the outer layer is the cuticle. The cuticle is the thin outer layer and is composed of transparent, overlapping, protective, scale-like cells pointing away from the scalp. This protects the inner structure of the hair but is sensitive to chemical, physical, and thermal damage. The middle layer is the cortex, which comprises 75-80% of the hair's protein structure. Imparts strength and elasticity to the hair strands and contains pigments (melanin), which impart colour to hair. Lastly is the medula, which is the central axis of the hair strand and comprises a column of soft cells containing air pocket.
Mainly, hair is composed of a protein called keratin. Hair also contains melanin (2%), lipids (up to 9%), and water (depends on the humidity.
Keratin is a polypeptide with monomeric repeat units consisting of most of the 20 amino acids with high proportions of glycine and alanine. One particular amino acid, cystine, accounts for 14-18% of keratin's structure. These cystine units give hair much of its strength by connecting parallel strands of protein chains to each another via covalent disulphide bonds. Besides in hair, keratin is also used to produce nails, claws, horns, hooves, and feathers.
 |
| The bonding in hair's protein chain |
Besides disulphide bonds, there are another three types of bonds (covalent, ionic, and hydrogen bonding) determine whether hair is straight or in loose waves ot tight curls. Although hydrogen bonding is weaker than ionic or covalent bonds, it actually gives dry hair most of its strength because it is so extensive.
When the hair is washed, the hydrogen bonds in keratin are disrupted by water and hair strands become more flexible. Blow-drying removes water and the keratin's hydrogen bonds reform, fixing the hair in its new shape. The mechanism of this process is:
 |
| Protein chain of hair in washing process |
- the adjacent protein chains in dry hair are held in place partly by hydrogen bonds.
- Hydrogen bonding with water 'swamps' these internal H bonds and allows hair protein chains to slide past each other when the hair strands are wet.
- Drying hair removes water and fixes its new orientation until hair gets wet again.
If the straight hair strand is turned into curly hair strands, ammonium thioglycolate and H2O2 can do that. Ammonium thioglycolate reduces disulphide links to thiol (-SH) groups so the hair strands can be separated from each other. Hence the hair strands and the thiol groups can be reoriented to form curly strands. Lastly, H2O2 oxidise some of the adjacent thiol groups to disulphide links to fix new orientation of the hair strands.
 |
| The mechanism of 'perms' (transforming straight hair into curly) |
Back to hair pigments, in natural there are two melanin pigments, eumelanin and pheomelanin. Eumelanine is a dark brown skin or hair pigment which is a complex polymer based on 5,6-dihydroxyindole. In the other sides, pheomelanin is a red-brown or yellow-brow pigment. Hence, the hair colour depends on the relative amounts of these two melanin pigments and the absence of these pigments produces white/grey hair. Moreover, it might be caused by build-up of H2O2.
Hair bleaches
In hair bleaches up to 12% H2O2 used to oxidise (i.e. bleach) the melanin pigments within the cortex, and it also destroys tyrosinase, the natural enzyme that helps to produce melanin. K2S2O8 is often used as a bleach accelerator and dilute dye solution act as 'toners'. However, overuse can cause severe chemical damage to hair strands, such as causes some disulphide cleavage and makes cuticles more porous.
 |
| The effect from overuse of hair bleaches |
Moreover, misuse can cause skin irritation, hair breakage, sensitisation, alopecia, etc.
Hair dyes
In fact, the hair colouring technology has been used back to the earliest records of human history and the deeply coloured vegetable extracts and powdered metals to colour hair (e.g. henna extract).
In general, there are 3 types of hair dyes, which will be discussed below.
Permanent hair dyes
Permanent hair-colouring consist of 2 components that are packaged separately and mixed immediately before application to the hair:
- 6% H2O2 aqueous solution
- Primary dye intermediates and 'couplers' in dilute NH3.
Couplers react with the oxidised primary dye intermediates for a wider colour range. Besides couplers, mixture of ammonia/dye solution and hydrogen peroxide solution is applied to the hair, which ammonia (less than 1%) causes hair strands to swell and cuticle scales to separate. Then, the dye precursors easily penetrate the cuticle before reacting with each other and H2O2 in situ. Once inside cuticle, polymerised dye is too large to diffuse out, so 'permanent' dye can be achieved.
 |
| Common chemical compounds in permanent hair dyes |
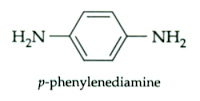
The hazardous of PPD (p-phenylenediamine) has been concerned in hair dye formulation. The manufacturer of PPD, DuPont, states that DuPont does not recommend and will not knowingly offer or sell p-phenylenediamine (PPD) for uses involving prolonged skin contact. Besides that, from 2007 British Medical Journal article stated allergic hair reactions are increasing as more people dye their and more than 2 in 3 hair dyes contain PPD. Hence, PPD is now banned in France, Germany, and Sweden due to its allergic reaction, but EU legislation still allows up to 6% PPD in a hair dye formulation. However, there are currently no satisfactory safe alternatives to PPD.
Semi-permanent hair dyes
 |
| Common dyes of semi-permanent hair dyes |
This dyes cannot lighten hair, since no oxidation involved and it is made from low molar mass dyes derived from coal tar that diffuse into cortex, so it last 4-6 weeks. Hence, it can easily diffuse to its low molar mass.
Temporary hair dyes.
 |
| Temporary hair dyes |
It has high molar mass, water soluble acidic dyes that simply adsorb onto the surface of the hair, rather than penetrate into cuticles. Hence, it can last for a week or two with a few shampoo washes.
Besides those hair dyes, there is another one which called 'anti-ageing' products. This products seem to use rather dubious chemistry to darken greying hair, which the activate ingredient in Grecian 2000 is lead acetate [Pb(CH3COO)2]. Lead acetate reacts slowly with the S-S bonds found in the cystine units of keratin. Then, after multiple applications, this leads to the formation of a thin layer of black lead sulphide (PbS) on the surface of the hair, but the toxicity of lead is questioned.
Shampoo
Generally, shampoo can be described as a concentrated solution of anionic surfactant at low pH (around 3-5). The anionic surfactant has a function to reduce the surface tension, cleans hair and scalp (removes oil, grease, and dirt). Hence, the surfactant exists in shampoo formulation as worm-like aggregates.
Besides that, many shampoos also contain chelating agent that sequester the metal ions (Ca2+ and Mg2+) in hard water, which would otherwise affect the surfactant performance.
Many shampoo formulations contain added 'conditioning agents' that replace at least part of the natural 'sebum' lubricant that is lost during the washing process and conditioning generally is a long chain alcohol. Besides surfactant and 'conditioning agent', there are another component such as pH adjustment, perfume, preservative, and additives.
As the cuticle is sensitive with chemical changes, the pH of shampoo formulation need to be adjusted to be suitable pH at hair environment. If hair is maintained at pH 4.5-5.5, cuticles are cationic to form compact and constricted cuticles. This structure protects the cortex and imparts sheen. However, at pH above 7, the cuticle is raised (get hair tangles, dullness) and inner cortex is exposed and more easily damaged. Hence, shampoos and conditioners are usually formulated to be mildly acidic (pH 3-5) to produce good lustre and allow hair strands to regain their optimum pH and the hair strands have maximum mechanical strength at this pH range.
 |
| The pH effect on the cuticles |
Shampoo formulation has been used for more than a century and since the earliest story of shampoo there are 3 major technical innovations in shampoo formulations:
- 1950's: replacement sodium stearate (soap) with synthetic sulphate-based surfactants.
- 1960's: introduction of cationic polymers as a 'conditioning agent' (lubricant).
- 1980's: use of polydimethylsiloxane (PDMS) as a 'conditioning agent', as well introduction of 2-in-1 formulations.
Conditioner
Generally, hair is much more fragile in its wet state due to reduced hydrogen bonding, so the conditioner address this problem. A conditioner has functions to reduce tangles, minimises hair damage, and prevents 'fly-away' hair by reducing static problems. Some conditioners contain various quaternary cationic surfactants, which are 'substantive' to anionic hair strands, so it get strong adsorption. Moreover, cationic surfactants are often used in combination with a fatty alcohol (e.g. n-hexadecanol or cetyl alcohol) to provide a 'softer feel'. Hence, the cationic surfactant and fatty alcohol binary mixture in a 'conditioning' shampoo forms a lamellar gel phase as shown below.
 |
| Lamellar phase structure of conditioner |
Furthermore, PDMS is also excellent conditioning lubricant for both wet and dry hair.
 |
| PDMS |
PDMS (dimethicone or silicone) is a hydrophobic liquid polymer with highly flexible chains. Besides that, PDMS is relatively expensive, but widely used in hair care formulations because of its excellent sensory benefits and water repellency (due to its hydrophobic characteristic). Moreover, PDMS has very low surface tension, which results in high spreadability on substrates like human hair and skin, so it is an extremely effective lubricant. Hence, deposition of PDMS makes hair look 'glossy' and feel 'silky'. In the other sides, it gives a new problem how to deliver PDMS into shampoo formulation which is an aqueous solution, meanwhile PDMS itself is a hydrophobic polymer.
To deliver PDMS in shampoo formulation, PDMS present as 1-20 μm droplets stabilised by anionic surfactant. 'Jaguar' is a high molecular weight cationic biopolymer (cellulose) based on 'guar gum'. The anionic surfactant micelles adsorb onto cationic 'jaguar' chains to form a so-called pearl necklace that renders 'jaguar' chains water soluble. Then, pearl necklace jaguar/surfactant complex adsorb onto and stabilises PDMS droplets.
 |
| PDMS stabilisation mechanism |
Then, on dilution (i.e. rinsing with water), cationic jaguar-anionic surfactant complex is destabilised and emulsion breaks, depositing PDMS onto hair strands. Besides that, cationic jaguar polymer also adsorbs onto anionic hairs strands to aid PDMS deposition and provides additional conditioning.
 |
| PDMS deposition process |
This patented PDMS deposition technology is used by 'Dove' shampoo (sold by Unilever as a 2-in-1 formulation), and other manufactures (P&G, L'Oreal, etc) use their own proprietary formulations.
 |
| 2-in-1 shampoo mechanism |
There are 3 main steps in 2-in-1 shampoo/conditioner delivers its function.
- The anionic surfactant cleans the hair just like a normal shampoo. Meanwhile, PDMS conditioning agent remains suspended in lather.
- On rinsing, the PDMS conditioning agent is released from suspension during dilution, while the oil and dirt are carried away.
- As with conventional conditioners, 2-in-1 products form a lubricious PDMS film on the hair surface that makes it feel softer and easier to comb.
Then, to increase the efficiency, there are important parameters that affect the PDMS deposition which include:
Physical properties of the guar gum
- Molecular weight
- Cationic charge density
- Polymer architecture
Hence, all of those are required the understanding in polymer science.
Physical properties of the PDMS droplets:
- Nature of the surface charge
- Particle size
- Viscosity (related to PDMS molecular weight)
- Therefore, the understanding in colloid or surface science is required.
In the other sides, too much PDMS deposition can make hair feel heavy, limp, and oily; and it affect the sales of 2-in-1 shampoos which have declined by 22% over 3 years due to adverse publicity. Hence, it need smaller PDMS droplets and optimum deposition for repeat sales
'Baby' Shampoo
Typical baby shampoo formulation:
Water, PEG-80 Sorbitan Laurate, Fragrance, Cocamidopropyl betaine, Sodium Trideceth Sulfate, Glycerin, Acrylates Copolymer, Amodimethicone, PEG-150 Distearate, Glycol Distearate, Steardimonium Hydroxypropyl Panthenyl PEG-7, Guar Hydroxypropyltrimonium Chloride, Tetrasodium EDTA, NaOH,Laureth-4, Dimethicone Phosphate Chloride,
PEG-14M, Quaternium-15, Hydrolysed Silk.
From the list of chemical compound there are 3 main points of baby shampoo. Firstly, in this formulation cocamidopropyl betaine is the surfactant which is a zwitterion surfactant, which in normal shampoo anionic surfactant is used. Secondly, Guar hydroxypropyltrimonium chloride is the polymer chain that stabilise PDMS (in this formulation is amodimethicone). Lastly is the hydrolised silk which is the oligopeptide chain and not necessarily from silk (it can be any protein sources). Furthermore, the acrylates copolymer is suggested to responsible for the appearance of shampoo.
Hair Spray
Hair spray is often as a simple formulation, which is essentially just a film-forming copolymer in ethanol/water mixture and a common film-forming that is used is poly(N-vinylpyrrolidone) or PNVP to give the spot welding. However, there are conflicting requirements for an effective hair spray:
- Deposition from an ethanol/water mixture
- Must be water-resistant (effect must no be ruined by high humidity)
- Must be easily removed by water (otherwise it get progressive build-up on hair)
Moreover, worldwide legislation-led drive to reduce VOC's has reduced ethanol content of hair spray from 88% to 55%, which inevitably leads to longer drying times. Lastly, according to P&G, there are 2 types of hair spray consumers with 50/50 market share:
- 'Flexible hold'
- 'Hold despite all odds'
Thus, it is difficult for any one hair spray product to satisfy all consumers.


















Comments